Medical Stoppers, Improved
TPEs offer excellent resealing, low levels of coring, and better manufacturing efficiency compared to traditional materials
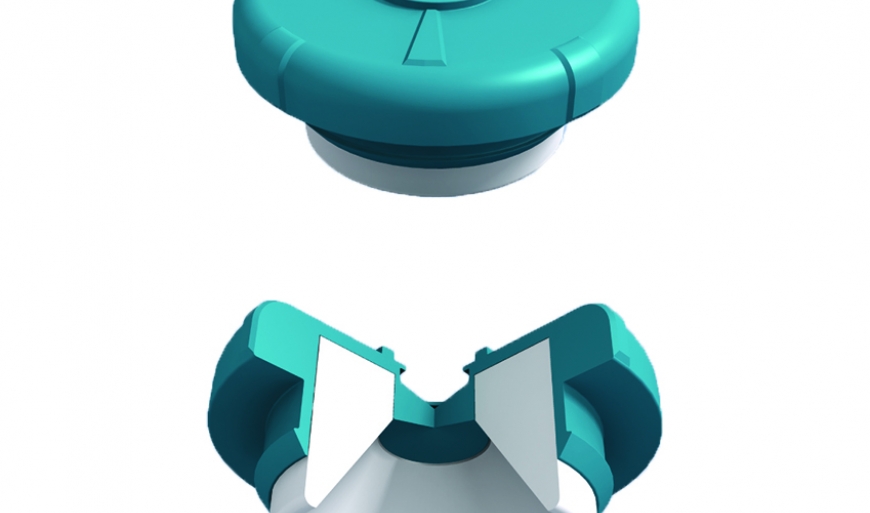
Stoppers and septums for medical infusion therapy systems must reseal after multiple penetrations. They also need to meet requirements for low levels of coring. Coring refers to the tiny fragments of material sheared off by the needle as it penetrates the stopper or septum, which could potentially contaminate the drug inside the container. Versaflex™ HC TPE is formulated to produce low coring levels in resealing applications. The material demonstrates good performance in testing with 6-mm infusion spikes and needles as large as 16 gauge - even after multiple penetrations. Because it meets this crucial requirement, the material can be used to streamline manufacturing and improve efficiency compared to traditional vulcanized rubber.
Fewer manufacturing steps, less time and cost
Versaflex HC processes in a simple, one-step injection molding operation instead of the multiple steps needed for vulcanized rubber, which requires raw materials to be premixed, processed for 5 -10 minutes on a two-roll mill, and made into strips to feed into a molding machine. The rubber is then injected into the mold and requires extra curing time. Afterward, parts need to be washed for 2 to 3 hours to remove unreacted crosslinking materials, such as zinc or sulfur. Plus, the washing agents require special disposal. With Versaflex HC, on the other hand, injection molding happens in seconds, and does not require curing or washing, thus saving significant time and reducing cost.
Strong bonds without an additional step
During the injection-molding step, the TPE insert forms a strong bond with the substrate, such as polypropylene or polyethylene, used for the container closure. Traditional vulcanized rubber products require application of adhesives to bond to the closure, and this adhesive bond has a greater potential for failure than the chemical bond formed between a TPE and polymer substrate. The adhesive is also an additional component of the container system that must be evaluated for compatibility before use.
Streamlined regulatory compliance
Versaflex HC grades give customers confidence that the materials will comply with regulatory requirements. This formulation meets the requirements of the ISO 15759 standard for medical infusion equipment, and can be steam autoclaved using temperatures up to 121°C. Purity and cleanliness are crucial for polymers used in healthcare applications, and this formulation enhances purity not only because it offers low coring levels, but because it is made without cross-linking agents that can potentially leach into the container and interact with the sensitive pharmaceutical product.